Introduction
Pre-transplant pulmonary dysfunction is known as an important risk factor for lung complications and subsequent non-relapse mortality (NRM) after allogeneic hematopoietic cell transplantation (allo-HCT). However, it remains unknown which cell source would be optimal for the patients with pre-transplant pulmonary dysfunction and whether the impact of cell source would differ according to recipient age, donor relation, and conditioning regimens. Therefore, we evaluated the impact of stem cell sources in patients with pre-transplant pulmonary dysfunction and in the subgroups.
Patients and methods
We performed a retrospective analysis of clinical data on 3374 allo-HCT in Japan between 2016 and 2020 using Japanese registry database. All patients had standard-risk disease and underwent their first allo-HCT from an HLA-matched donor. Their clinical outcomes were compared between peripheral blood stem cells (PBSCs) and bone marrow (BM) grafts individually in two distinct cohorts, namely the Lung-scored (LS) cohort and the normal cohort, based on the presence of lung scores determined by the Hematopoietic Cell Transplantation-specific Comorbidity Index. In addition, we conducted subgroup analyses to explore potential differences in the impact of cell sources on these outcomes according to the recipient age (≥50 or < 50), donor relation (related or unrelated), and conditioning regimens (cyclophosphamide [CY] / total body irradiation [TBI]-based myeloablative conditioning [MAC], busulfan [BU]-based MAC, or reduced-intensity conditioning [RIC]).
Results
In the LS cohort, the 2-year overall survival (OS) tended to be higher in the BM group than that in the PBSCs group (71.9% vs 61.4%; P = 0.052). However, in the normal cohort, there was no significant difference between both the cell source types (71.6% vs 73.1%; P = 0.13). Multivariate analyses showed that PBSCs were significantly associated with inferior OS in the LS cohort (hazard ratio [HR], 1.60 [95% CI, 1.06-2.42], P = 0.026). On the other hand, cell source type did not significantly affect OS in the normal cohort (HR, 0.94 [95% CI, 0.78-1.14], P = 0.55). We also found that PBSCs were significantly associated with an increased risk of NRM in the LS cohort (HR, 1.90 [95% CI, 1.03-3.52], P = 0.041), while cell source types did not affect NRM in the normal cohort (HR, 0.85 [95% CI, 0.64-1.13], P = 0.26). PBSCs were not identified as a risk factor for relapse in both cohorts.
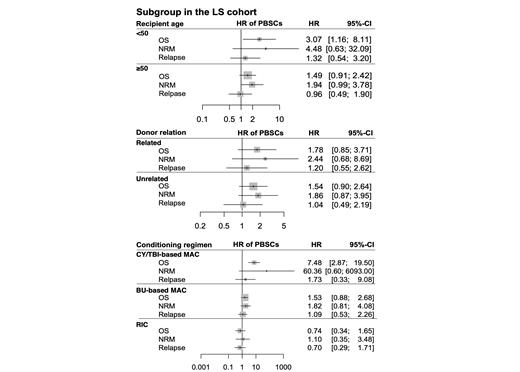
When we focused on the LS cohort alone, the Figure 1 summarized the impact of the use of PBSCs in the subgroups according to age, donor relation, and conditioning regimens. Briefly, the use of PBSCs exhibited a significant adverse impact on OS (HR, 3.07 [95% CI, 1.16-8.11], P = 0.024) in patients younger than 50 years old. For recipients aged 50 years or older, PBSCs were not associated with inferior OS (HR, 1.49 [95% CI, 0.91-2.42], P=0.11), although it tended to be associated with an increased risk of NRM with a borderline significance (HR, 1.94 [95% CI, 0.99-3.78], P = 0.053, Figure 1). Focusing on the subgroup stratified according to their donor relation, the use of PBSCs were not associated with inferior OS or an increased risk of NRM in both allo-HCT groups from a related donor or unrelated donor. Furthermore, focusing on the subgroups stratified according to conditioning regimens, the use of PBSCs had a significantly negative impact on OS in the subgroup with CY/TBI-based MAC (HR, 7.48 [95% CI, 2.87-19.51], P < 0.001). On the other hand, the use of PBSCs had no significant impact on OS or NRM in the subgroups of BU-based MAC or RIC. Regarding relapse incidences, the impacts of the use of PBSCs were not different in any of the individual subgroups.
Conclusion
The use of PBSCs were associated with inferior survival in the LS cohort, but not in the normal cohort. When we focused on the subgroups of the LS cohort alone, the use of PBSCs were associated with inferior survival in the subgroup of younger recipients or those with CY/TBI-based MAC. These patients in the LS cohort might benefit from the use of BM. These results should be validated in another registry study, and further research would be warranted for the purpose of preventing adverse impact of PBSCs or the optimal cell source selection.
Disclosures
Kawamura:Nippon Shinyaku: Speakers Bureau. Tamaki:Chugai Pharmaceutical: Honoraria. Sakaida:Pfizer: Consultancy, Speakers Bureau; Otsuka: Consultancy; Ohara: Consultancy; Human Life Cord Japan: Consultancy; Chugai: Research Funding; Kyowa-Kirin: Research Funding; Takeda: Speakers Bureau; Janssen: Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau. Hayashi:Sanofi: Honoraria. Doki:Janssen Pharmaceutical K.K.: Honoraria; Novartis Pharma K.K.: Honoraria. Sawa:Sanofi: Honoraria; Janssen: Honoraria. Tanaka:Astellas Phrama: Speakers Bureau; Chugai Pharmaceutical: Speakers Bureau; Daiichi Sankyo: Speakers Bureau; Kyowa-Kirin: Speakers Bureau; MSD: Speakers Bureau; Otsuka Pharmaceutical: Speakers Bureau; Pfizer: Speakers Bureau; Sumitomo Pharma: Speakers Bureau; Asahi Kasei Pharma: Speakers Bureau; Abbvie: Speakers Bureau. Atsuta:Meiji Seika Pharma Co, Ltd.: Honoraria; Otsuka Pharmaceutical Co., Ltd: Speakers Bureau; JCR Pharmaceuticals Co., Ltd.: Consultancy; Novartis Pharma KK: Speakers Bureau; CHUGAI PHARMACEUTICAL CO., LTD.: Speakers Bureau. Kanda:Human Life CORD: Speakers Bureau; Sumitomo Pharma: Research Funding, Speakers Bureau; Amgen: Speakers Bureau; Takeda Pharmaceutical: Research Funding, Speakers Bureau; Meiji Seika Pharma: Speakers Bureau; Asahi Kasei Pharma: Research Funding, Speakers Bureau; Daiichi Sankyo: Research Funding, Speakers Bureau; Saitama Hokeni Kyokai: Speakers Bureau; MSD: Speakers Bureau; Kyowa Kirin: Research Funding, Speakers Bureau; Janssen Pharmaceutical: Speakers Bureau; Sanofi: Speakers Bureau; Pfizer: Speakers Bureau; Chugai Pharmaceutical: Research Funding, Speakers Bureau; Novartis: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Otsuka Pharmaceutical: Research Funding, Speakers Bureau; AstraZeneca: Speakers Bureau; Japan Blood Products Organization: Research Funding, Speakers Bureau; CSL Behring: Speakers Bureau; AbbVie: Research Funding, Speakers Bureau; Towa Pharma: Speakers Bureau; Precision: Speakers Bureau; Eisai: Research Funding, Speakers Bureau; Nippon Shinyaku: Speakers Bureau; FUJIFILM Wako Pure Chemical: Speakers Bureau; Shionogi Pharma: Research Funding; Wakunaga Pharmaceutical: Speakers Bureau; Alexion Pharma: Speakers Bureau; Taiho Pharmaceutical: Research Funding; Nippon Kayaku: Research Funding; JCR Pharmaceuticals: Research Funding. Yakushijin:Janssen Pharmaceutical: Honoraria; Novartis: Honoraria; Asahi Kasei Pharma: Honoraria; Otsuka Pharmaceutical: Honoraria; Pfizer: Honoraria; Jazz Pharmaceuticals: Honoraria; Nippon Shinyaku: Honoraria; Astrazeneca: Honoraria; Chugai Pharmaceutical: Research Funding. Kanda:CHUGAIIGAKU CO., LTD.: Honoraria; TERUMO CORPORATION: Honoraria; DAIICHI SANKYO Co., Ltd.: Honoraria; Astellas Pharma Inc.: Honoraria; Kyowa Kirin Co., Ltd.: Honoraria; Wakunaga Pharmaceutical Co., Ltd.: Honoraria; Bristol-Myers Squibb Co: Honoraria; Novartis Pharma K.K.: Honoraria; ASAHI KASEI PHARMA CORPORATION: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Amgen Pharma Inc.: Honoraria; Nippon Shinyaku Co., Ltd.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; CSL Behring K.K.: Honoraria; NIPPON KAYAKU CO. LTD.: Honoraria; MSD K.K.: Honoraria; asclepia: Honoraria; AbbVie Inc.: Honoraria; Megakaryon Co: Consultancy; Sanofi K.K.: Honoraria; CHUGAI PHARMACEUTICAL Co., Ltd.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Fujimoto Pharmaceutical Corporation.: Honoraria; Eisai: Research Funding. Nakasone:Takeda Pharmaceutical: Honoraria; Sanofi: Honoraria; Chugai Pharmaceutical: Honoraria; Eisai: Honoraria; Meiji Seika Pharma: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Bristol-Myers Squibb: Honoraria; Otsuka Pharmaceutical: Honoraria; Nippon Shinyaku: Honoraria.